-
Whitepaper
TAP® technology shows promise for early, precise Alzheimer's monitoring via biomarkers
HIGHLIGHTS
- The TAP is a non-invasive and virtually painless blood collection device that enables point-of-care, high-quality whole blood collections for downstream molecular applications such as automated point-of-care SNP (single nucleotide polymorphism) genotyping.
- The Cepheid GeneXpert FII and FV FDA-approved IVD test for genetic risk of thrombosis enables an automated solution for an end-to-end workflow (sample to report) in 30 minutes via interrogation of the FII and FV genes at specific nucleic acid positions of 20210 G/A and 1691 G/A, respectively.
- Assessment of quality controls, cycle threshold (Ct) values, and fluorescence endpoints (EndPt) for SNP polymerase chain reaction (PCR) data demonstrate comparable equivalence between TAP vs traditional venipuncture blood collections, yielding 100% concordance of SNP calls and 100% pass rate for QC criteria within the FDA-approved assay and instrument.
- Applications for efficient blood collection coupled to rapid molecular testing platforms enable on-demand global population testing at point-of-care for physician offices, clinical sites and remote/underserved areas.
INTRODUCTION
Factor II (G20210A) and Factor V Leiden (G1691A) mutations are associated with an increased risk for venous thrombosis. Factor II c.*97G>A was previously designated as G20210A or 20210G>A4 and is commonly referred to as prothrombin (or in the Xpert Factor II & Factor V test as Factor II G20210A). The Factor II (G20210A) mutation refers to the G to A transition at nucleotide 20210 in the 3′ untranslated region of the gene and is associated with increased plasma levels of prothrombin. Factor V c.1601G>A (p.Arg534Gln) was previously designated as G1691A or Arg506Gln and is commonly referred to as Factor V Leiden or FVL (or in the Xpert Factor II & Factor V test as Factor V G1691A). Factor V Leiden (G1691A) refers to the G to A transition at nucleotide position 1691 of the Factor V gene, resulting in the substitution of the amino acid arginine by glutamine in the Factor V protein, causing resistance to cleavage by Activated Protein C (APC). Factor II (G20210A) and Factor V Leiden (G1691A) mutations are present in 2% and 5% of the general population, respectively. The Xpert Factor II & Factor V Assay is a qualitative in vitro diagnostic genotyping test for the detection of Factor II and Factor V alleles from citrated or EDTA anticoagulated whole blood. The test is performed on the Cepheid GeneXpert System. This test is intended to provide results for Factor II (G20210A) and Factor V Leiden (G1691A) mutations as an aid in the diagnosis in individuals with suspected thrombophilia.
Traditional blood collection for the Cepheid GeneXpert system involves a venipuncture by a trained phlebotomist, whereby 5-10 ml of whole blood is collected in a standard citrate/heparin/EDTA tube. This procedure is relatively costly and inaccessible to scale across large patient populations for point of care testing. Economic and logistical hurdles to high-quality blood collections persist due to the modality of the sample collection process (primarily due to the need of a trained phlebotomist/technician), thereby impeding access to many people for efficient point-of-care testing modalities such as the Cepheid GeneXpert system.
Here, we introduce the Yourbio Health TAP blood collection device as a viable tool to enable feasibility of blood collection scale and adoptability for downstream molecular testing on the Cepheid GeneXpert system. The TAP enables point-of-care collections by novice users, direct use by physicians/technologists in general practices, or collections in underserved/remote geographies. We obtained paired blood samples (TAP + traditional venipuncture) to analyze downstream Cepheid data to demonstrate analytical concordance verification of genotype calls and quality metrics. We assess the Ct, fluorescence endpoints, and QC metrics.
MATERIALS AND METHODS
Under an IRB-approved protocol, twelve donors had paired blood samples collected by a trained phlebotomist (traditional venipuncture tube) and self-collected (TAP device) into heparin-coated tubes. The venipuncture tubes collected the full max fill volume of 3 ml by the phlebotomist, whereas the TAP devices collected a minimum of 300 ul and maximum of 600-800 ul of whole blood. All blood samples were collected and treated identically (room temperature storage and ambient transit) in preparation for downstream Cepheid GeneXpert testing. Briefly, 50 ul of each sample collected from both methods were pipetted into the Cepheid GeneXpert cartridge, whereby GeneXpert System automates and integrates sample purification, nucleic acid amplification, and detection of the target sequence in whole blood using real-time PCR assays. The system requires the use of single-use disposable cartridges that hold the PCR reagents and conduct the PCR process. Since the cartridges are self-contained, cross-contamination between samples is eliminated. The Xpert Factor II & Factor V Assay includes reagents for the detection of Factor II and Factor V normal and mutant alleles, whereby each assay cartridge also contains a Probe Check Control (PCC) that verifies reagent rehydration, PCR tube filling in the cartridge, probe integrity, and dye stability. The primers and probes in the Xpert Factor II & Factor V Assay determine the genotype of the Factor II gene (at position 20210) and/or the Factor V gene (at position 1691). Automated results are reported in 30 minutes.
We assessed the Ct values and the endpoint fluorescence, whereby Ct values were used as the primary assessment for variance analysis. Endpoint fluorescence was simply used as a qualitative indicator for amplification (generally greater than the controls “no call” values), whereas Ct values should demonstrate low variance between the two sample types given that the extraction, amplification and instrument cycling procedures are standardized, automated and completely controlled within the Cepheid testing cartridge and instrument.
RESULTS
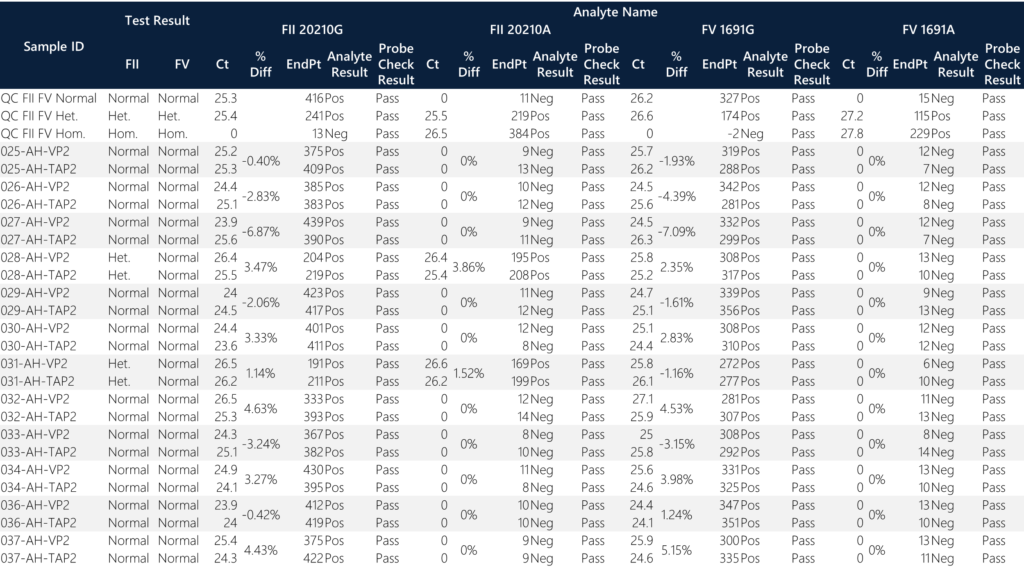
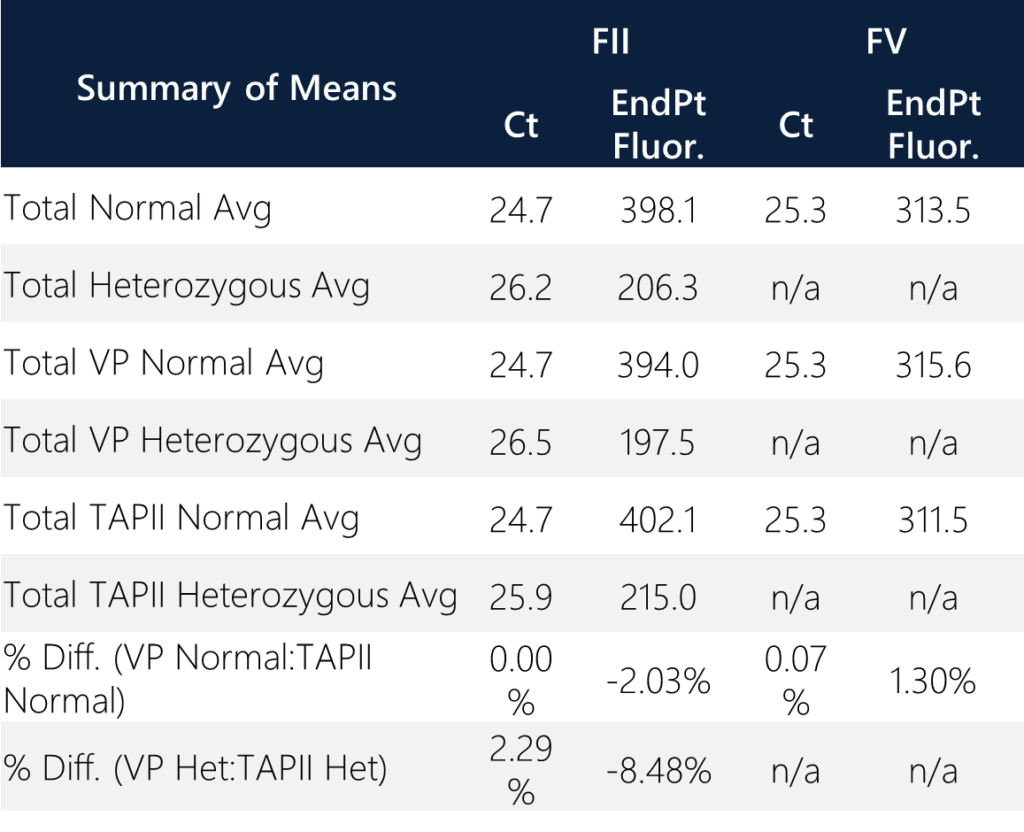
All samples passed the probe QC check and produced an analyte result. When screening for FII/FV variants, the percentage difference between all donor pairs are less than 7%. All twelve donors are normal for FV status while two donors (28 and 31) are heterozygous for FII status. None of the donors are homozygous for FII/FV. All donors are normal for FV status. Donors 28 and 31 exhibit ~50% endpoint fluorescence as compared to the normal donors for FII status. This is expected since half of the total alleles are represented in the heterozygous state for 20210G/A variant positions. When compared to normal individuals for FII status with total average endpoint fluorescence = 398.1, the two heterozygous samples have a total average endpoint fluorescence of 206.3 (approximately 51.8%). In general, this is a good qualitative QC check for heterozygous samples. The overall average “no call” endpoint fluorescence for FII/FV status is 10.6. The overall average normal endpoint fluorescence for FV status is 313.5.
Summary of the means were assessed between sample types and variant calls. The percent difference of average Ct values between TAPII vs. VP for normal and heterozygous samples are <0.07% and <2.3%, respectively. The percent difference of the average endpoint fluorescence between TAP vs VP for normal and heterozygous samples are <2.03% and <8.48%, respectively. Overall, the quantitative results correlate with the qualitative amplification calls and the reporting outcomes. (Note: homozygous samples, excluding the control, were not identified within the sample set.)
CONCLUSIONS
Initial feasibility for the Cepheid GeneXpert FII/FV assay assay demonstrates 100% call concordance between TAP and VP samples. Specifically, the Ct and EndPt values show low percentage differences between the overall averages of TAP vs. VP. Accessible and minimally invasive sampling with the TAP device can enable rapid and hands-off utility for monitoring needs. Remote collections with TAP can enable access and rapid point of care diagnostics when coupled to closed-system platforms such as the Cepheid GeneXpert cartridge-based platform. The purpose of this study is to demonstrate technology and platform capability of the TAP device with the GeneXpert system. Further evaluation of the TAP device could benefit from a larger sample size and comparisons to similar orthogonal assessments (for example, real-time PCR). Future studies will explore screening applications with large populations for patient stratification and diagnostic potential. Additional considerations may include expansion of the GeneXpert assays to incorporate additional analytes.