Longer interval between maternal RSV vaccination and birth increases placental transfer efficiency
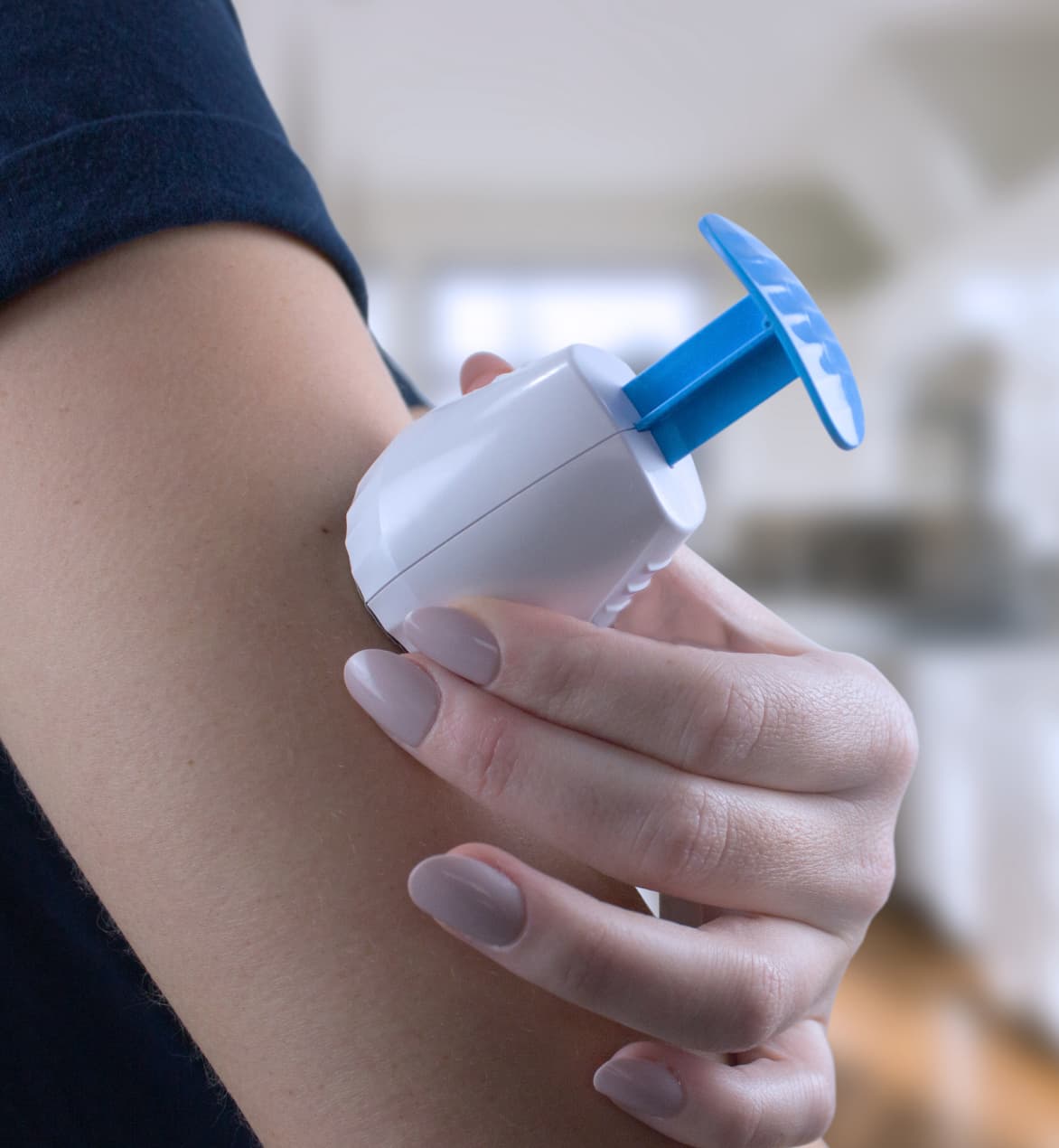
TAP was employed to collect capillary blood from 2-month-old infants for antibody tests of RSV and pertussis toxin.
Read More
TAP® devices take the pain out of decentralized clinical trials
Our Touch Activated Phlebotomy (TAP®) devices expand decentralized clinical trial accessibility by enabling easy and reliable collection of up to 900µL of high-quality, whole capillary blood, both remotely and in clinical settings, without the need for phlebotomists or painful fingersticks.
The cost and inconvenience of travel to a clinical trial site have historically been detrimental to participant enrollment. For some patient populations, such as immunocompromised individuals, those with rare diseases who can be geographically widespread, and those with significant mobility issues, trial enrollment can be very difficult and derail the study’s progress.
Decentralized clinical trials help sponsors avoid these barriers while also helping to expand clinical trial catchment, increasing participant diversity and increasing participation for traditionally hard-to-access patient populations, thus providing data that is more representative of the ultimate patient population.

YourBio Health’s comprehensive decentralized clinical trial service ensures easy management and tracking of patient samples that fits seamlessly into your trial protocol and supports the recruitment of diverse and representative patient cohorts. We recognize each clinical trial is unique and can modify our services to fit your specific study.
Our range of services includes:



For broad-based tests, such as cholesterol, capillary blood samples collected in both an assisted and self-collection setting have high concordance with venous blood.6
Traditional blood draw methods like standard needles and lancets cause substantial pain and inconvenience to many patients and pose a significant deterrent to testing in some patient populations. Our TAP® devices, TAP® Micro and TAP® Micro Select, overcome these barriers thanks to the microneedle array of our HALO™ Technology which allows for virtually painless, bladeless blood draws.